Chapter 4: Beginner’s guide to the Retina
The retina can be intimidating as it’s not easy to visualize the posterior pole and there is a bunch of pathology back there. There are many things I could cover in this chapter, but I’ve decided to keep things simple and only discuss a few topics like diabetic retinopathy and retinal detachments. Other disease processes that involve the retina will be covered in other chapters.
Diabetic Retinopathy
Diabetes is a common disease and many affected patients have vision problems. In fact, diabetics are twenty times more likely to go blind than the general population. Diabetic retinopathy is the term used to describe the retinal damage causing this visual loss. Diabetics have a high prevalence of retinopathy, and one out of every five patients with newly diagnosed diabetes will also show signs of retinopathy on exam.
Mechanism of Vessel Breakdown
How are the eyes affected? Basically, diabetes is a disease of blood vessels. With large amounts of glucose coursing through the circulatory system, a glycosylation reaction occurs between the sugar and the proteins that make up the vessel walls. Over time, this glycosylation promotes denaturing of collagen protein within the walls, creating capillary thickening and eventually, wall breakdown.
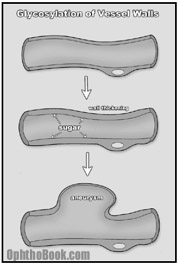 While this process occurs throughout the entire body, the microvasculature of certain organs, such as the kidneys and eyes, are more susceptible to damage. Along these lines, a good predictor of microvasculature damage in the diabetic eye is prior evidence of renal microvasculature disease as measured by proteinurea, elevated BUN, and creatinine.
While this process occurs throughout the entire body, the microvasculature of certain organs, such as the kidneys and eyes, are more susceptible to damage. Along these lines, a good predictor of microvasculature damage in the diabetic eye is prior evidence of renal microvasculature disease as measured by proteinurea, elevated BUN, and creatinine.
Because vessel damage accumulates over time, the most accurate predictor of retinopathy is duration of diabetes. After 10 years, more than half of patients will show signs of retinopathy, and after 15 years this number increases to nearly 90%. The relative control of glucose during this time is also important, and studies have shown that patients who maintain lower hemoglobin A1C levels have delayed onset and slower progression of disease. Additional risk factors include smoking, hypertension, and pregnancy.
Two Types of Retinopathy
It is useful to divide patients into two categories of retinopathy.
A. Nonproliferative diabetic retinopathy (NPDR)
Most patients (95%) have NPDR. This is the earliest stage of retinopathy and it progresses slowly. Because so many diabetic patients have NPDR, this stage is commonly described as “background retinopathy.” The earliest signs of retinal damage arise from capillary wall breakdown, seen on the fundus exam as vessel microaneurysms. Injured capillaries can leak fluid into the retina and the aneurysms themselves can burst, forming “dot-and-blot hemorrhages.”
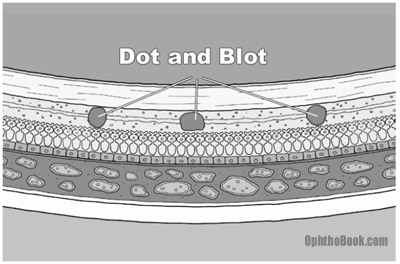
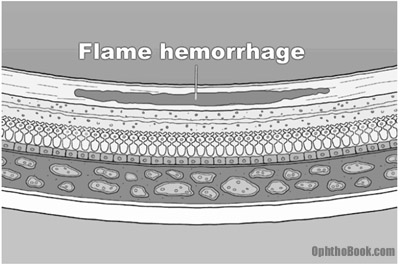
Dot-blot hemorrhages look small and round because they occur in the deep, longitudinally-oriented cell layers of the retina. This contrasts with the “flame hemorrhages” of hypertension that occur within the superficial ganglion nerve layer, and thus spread horizontally.
With worsening retinopathy and vessel damage, the retina begins to show early signs of ischemia. Cotton-wool spots, seen with hypertension and stasis, are gray spots with soft edges that indicate ischemia/infarction of the superficial retinal nerve fibers. As vessel damage progresses, you can also see beading of the larger retinal veins and other vascular anomalies.
B. Proliferative Retinopathy
With ongoing injury to the retinal vasculature, there eventually comes a time when the vessels occlude entirely, shutting down all blood supply to areas of the retina. In response, the ischemic retina sends out chemicals that stimulate growth of new vessels. This new vessel growth is called neovascularization, and is the defining characteristic of proliferative retinopathy. Far fewer patients have proliferative retinopathy, which is fortunate as this stage can advance rapidly with half of these patients going blind within five years if left untreated. The mechanism and complications of neovascularization merit study, so let’s take a closer look.
The Mechanism of Neovascularization
With complete vessel occlusion, parts of the retina become starved for nourishment. The ischemic retina responds by releasing angiogenic molecules like VEGF to promote new vessel growth. These new vessels serve to bypass the clogged arteries in order to resupply the starved retina.
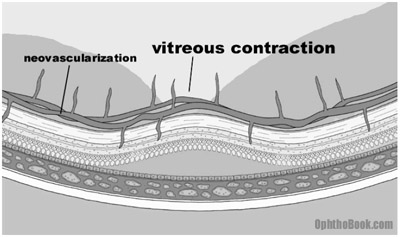
A collateral blood supply seems like a great idea, but unfortunately there is a problem. The newly formed vessels are abnormal in both appearance and function. The new vessels are friable and prone to leaking. They also grow in the wrong place, spreading and growing along the surface of the retina. They can even grow off the retina, sprouting up into the vitreous fluid. The vitreous is mostly water, but it also contains a lattice framework of proteins that the new vessels can adhere to. With vitreous movement or contraction, these new connections pull on the retina and the traction can lead to retinal detachment. Since the vessels are also weak, any vitreous traction can break the vessels and create sudden hemorrhaging with subsequent vision loss as the eye fills with blood. Finally, the new vessels can regress and scar down, creating massive traction on the retina underneath.
Neovascularization isn’t just limited to the retina, but can also occur on the iris itself. NVI (neovascularization of the iris) is an ominous sign, as the new vessels can cover the trabecular meshwork and create a sudden neovascular glaucoma.
Macular Edema
Despite the neovascularization phenomenon and its potential for detachments and hemorrhage, the most common cause of blindness in diabetic patients is from macular edema. This occurs when diffuse capillary and microaneurysm leakage at the macula causes the macular retina to swell with fluid.
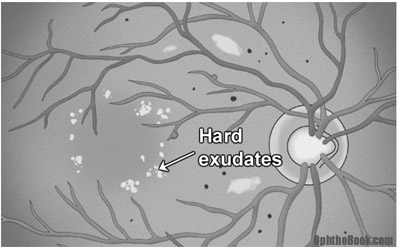
Macular edema occurs in about 10% of patients with diabetic retinopathy and is more common with severe retinopathy. On exam the macula looks cloudy and mildly elevated, and you can see past evidence of edema in the form of yellow-colored “hard exudates”. These exudates are fatty lipids that are left behind after past macular swelling subsides, similar to a dirt ring in a bathtub.
Treatment of DR (diabetic retinopathy)
Preventative medicine with tighter control of glucose is the ideal treatment, but for worsening symptoms, surgical treatment is necessary. The two main surgeries are laser treatment and vitrectomy.
Laser Treatment
 In cases of macular edema, an argon laser can be used to seal off leaking vessels and microaneurysm in the retina by burning them. If the leakage or microaneurysm is small and well-defined, it can be selectively sealed off. With larger areas of leaking capillaries, such as diffuse macular edema, the laser can lay down a “grid photocoagulation” pattern over the entire area.
In cases of macular edema, an argon laser can be used to seal off leaking vessels and microaneurysm in the retina by burning them. If the leakage or microaneurysm is small and well-defined, it can be selectively sealed off. With larger areas of leaking capillaries, such as diffuse macular edema, the laser can lay down a “grid photocoagulation” pattern over the entire area.
With advanced retinopathy and neovascularization, a different approach is taken. Instead of individually targeting vessels, PRP (pan-retinal photocoagulation) is performed. With PRP, the ophthalmologist burns thousands of spots around the peripheral retina. This destroys the ischemic retina, decreasing the angiogenic stimulus, and commonly leads to regression and even the complete disappearance of the new vessels. This treatment may seem drastic, but it has proven to be effective. Naturally, there are side effects, with peripheral vision loss and decreased night vision (from the rod photoreceptor loss), but this is acceptable if the central vision is saved. I’ve never seen anyone actually complain of decreased vision, but it’s possible and should be stressed during consent.
Vitrectomy
A vitrectomy may also need to be performed and is often done in conjunction with other surgeries. This surgery involves removing the vitreous humor from the eye and replacing it with saline. This allows removal of hemorrhaged blood, inflammatory cells, and other debris that may obscure the visual axis. While removing the vitreous, the surgeon also removes any fine strands of vitreous attached to the retina in order to relieve traction that might have, or will, cause a detachment.
Conclusion
As you can see, diabetic retinopathy is a big problem as a large percentage of our patients have diabetes. Retinal vessel damage leads to edema, and vessel occlusion stimulates neovascularization that can lead to trouble. Fortunately, better glucose control and surgical treatments have significantly decreased the incidence of visual loss in these patients.
Retinal Detachments
A retinal detachment is an abnormal separation between the sensory retina and the underlying RPE and choroid plexus. If you remember from the anatomy lecture, the outer third (the part furthest from the vitreous) of the retina gets its nourishment primarily from the underlying choroid – with a detachment, the photoreceptor layer loses its blood supply and becomes ischemic. The macular retina is especially susceptible to this damage. The prognosis for patients with retinal detachments depends upon the quickness to treatment; patients with detachments that involve the macula have much worse outcomes.
Risk Factors and Epidemiology
Up to six percent of the general population have retinal breaks of some kind, though most of these are benign atrophic holes. The actual incidence of retinal detachment is only 1 in every 10,000 people. Relative risk is equal between men and women, with higher rates in those of Jewish descent and decreased risk in black populations.
When looking at patients who already have retinal detachments, you begin to see some interesting trends. Many of these patients are myopic (near-sighted). Myopic eyes are physically larger and longer than normal eyes and have thinner retinas at the periphery. This thin retina is more likely to break, forming small holes and tears that may progress to a detachment.
Up to 35 percent of patients with retinal detachments develop them after another eye surgery – typically a cataract extraction. Finally, traumatic sports such as boxing, football, and bungee-jumping predispose younger people to forming detachments.
The Three Types of Detachment
Retinal detachments generally occur by three different mechanisms.
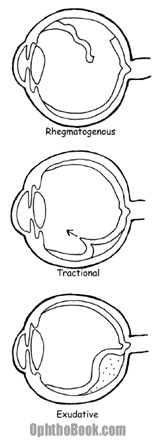 1. The most common detachment is the rhegmatogenous retinal detachment. This is an actual tear in the retina, with a full-thickness break through the retinal sensory layers. These tears can occur from trauma, surgery, or extend from preexisting retinal holes. Fluid from the vitreous chamber flows through the tear and collects in the sub-retinal space. Eventually, the retina tears away, peeling off the underlying RPE and choroid. Without treatment, a rhegmatogenous detachment can spread and eventually involve the entire retina.
1. The most common detachment is the rhegmatogenous retinal detachment. This is an actual tear in the retina, with a full-thickness break through the retinal sensory layers. These tears can occur from trauma, surgery, or extend from preexisting retinal holes. Fluid from the vitreous chamber flows through the tear and collects in the sub-retinal space. Eventually, the retina tears away, peeling off the underlying RPE and choroid. Without treatment, a rhegmatogenous detachment can spread and eventually involve the entire retina.
2. The second type of detachment is from traction on the retina. This is when the retina is pulled from its base. This can occur from vitreous pulling, or from diseases like diabetic retinopathy where neovascular membranes on the retinal surface contract and tug on the retina with great force.
3. A less common mechanism for detachment is from hemorrhagic or exudative retinal detachment. This occurs when blood or fluid builds up under the retina, slowly pushing the retina upwards. This occurs with dysfunction of the RPE or choroid plexus and can be caused by ocular tumors, inflammatory diseases, or congenital abnormalities that create a breakdown of the blood-retina barrier.
PVD (posterior vitreous detachment)
One common cause of a retinal tear is secondary to a posterior vitreous separation. As we age, the vitreous liquefies and contracts in upon itself … if this occurs suddenly, the posterior vitreous face can suddenly peel off the retina. Usually, this isn’t a problem, but if the vitreous is abnormally adherent to the retina the separation may rip a small hole in the retina that progresses into a detachment.
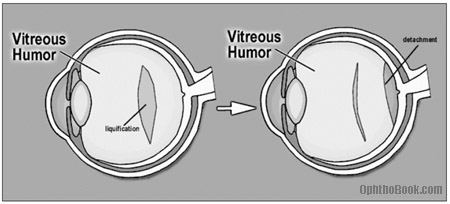
PVDs are very common in people over 65 and are a major source of “annoying floaters” in this population. We check these patients very closely, and, assuming no tears are seen, check them again in a few weeks to insure none have developed.
Symptoms
With detachment, patients often report seeing flashes of light and floaters. Flashing lights, or photopsias, are often seen when a detachment first occurs. Photoreceptors are normally triggered by light, but severe mechanical disturbance can stimulate them as well giving the sensation of light like a camera flash.
Floaters look like dark specks that obscure vision, and patients say they look like a swarm of flies. They are created by objects (blood cells or pigment) floating in the vitreous fluid that cast shadows on the retina. While the presence of a few floaters is normal, the sudden appearance of hundred of floaters may indicate a vitreous hemorrhage.
Another symptom that is sometimes described is seeing a “dark curtain” that obscures peripheral vision, as most detachments occur in the peripheral retina. The combination of flashing lights and floaters should be considered a retinal detachment until proven otherwise.
Findings
The definitive way to diagnose a retinal detachment is to actually see it with the indirect ophthalmoscope. If the tear is large enough, it will be obvious as the floating retina contains blood vessels and undulates with eye movement. Suspended pigment particles may be seen floating in the anterior vitreous (Shafer’s sign) that is described as “tobacco dust,” and is pathognomonic for a retinal tear.
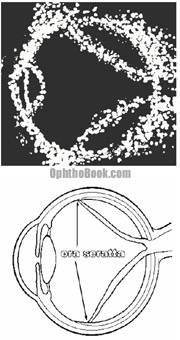 An ultrasound of the eye may be helpful, especially when the tear is not obvious or when the retina can’t be visualized because of hemorrhaging or cataracts. An ultrasound can also pick up other pathology such as tumors.
An ultrasound of the eye may be helpful, especially when the tear is not obvious or when the retina can’t be visualized because of hemorrhaging or cataracts. An ultrasound can also pick up other pathology such as tumors.
This illustration shows an ultrasound of a patient with a complete retinal detachment. The retina looks like a letter V in this picture, because it is still attached at two places – the optic disk and at the peripheral ora seratta. Choroidal effusions can give a similar appearance, but I won’t talk about them because it would just be confusing at this point.
Treatment Options
The treatment for retinal detachment varies. The primary treatment for the majority of retinal tears and traction detachments is surgical. How fast a patient needs surgery depends upon whether the central macula has detached or not. If the macula has detached, then the vision is pretty much toast, so it may be ok to wait a few days before going to surgery. If the macula is still on, then you want to make sure it STAYS on, so you go to surgery sooner.
If the retina has a tear or hole that hasn’t yet detached, the tear can be “pegged down” by welding down the surrounding retina with a laser. The retina can also be scarred down by freezing it into place with a cryoprobe applied from the outside of the eye.
Scleral buckling is the traditional surgical procedure, and involves encircling the eye with a silicone band that squeezes the eye like a belt. The buckle indents the eye and pushes the RPE into contact with the retina, allowing it to heal into place. Because of the orbital anatomy, scleral buckles are most useful for anterior breaks at the equator because you can’t really buckle the back of the eye.
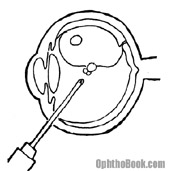 Over the past few decades, pneumatic retinopexy has become quite popular. In this procedure, after repairing the retinal tear the surgeon injects a bubble of gas or silicon oil into the globe which acts to push (or tamponade) the retina into position until it heals. There are many different types of gas that we use, but they all eventually absorb back into the body. The disadvantage to this procedure is that patients have to keep their head down for several weeks to keep the bubble in place. This is very taxing and patients tend to look quite disheveled at their post-op appointments. An oil bubble doesn’t require this head positioning, but does require a return to the OR to remove the oil.
Over the past few decades, pneumatic retinopexy has become quite popular. In this procedure, after repairing the retinal tear the surgeon injects a bubble of gas or silicon oil into the globe which acts to push (or tamponade) the retina into position until it heals. There are many different types of gas that we use, but they all eventually absorb back into the body. The disadvantage to this procedure is that patients have to keep their head down for several weeks to keep the bubble in place. This is very taxing and patients tend to look quite disheveled at their post-op appointments. An oil bubble doesn’t require this head positioning, but does require a return to the OR to remove the oil.
If the detachment is severe and complicated, a vitrectomy may need to be done. The vitreous fluid is removed, and the retina is manually floated back into position. With access to the inner globe, scar tissue and any other causes of traction, such as the neovascular membranes, can be removed.
Rubber Band Theory
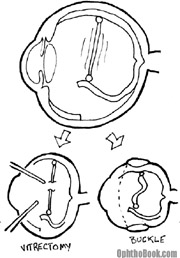 When treating a retinal detachment, a good way to think about traction is the “rubber band” theory. Thus, there is almost always some tension inside the eye that is keeping the retina from laying flat. There are two ways to relieve this tension: you can perform a vitrectomy and “cut” the band, or you can perform an encircling buckle procedure to shorten the band.
When treating a retinal detachment, a good way to think about traction is the “rubber band” theory. Thus, there is almost always some tension inside the eye that is keeping the retina from laying flat. There are two ways to relieve this tension: you can perform a vitrectomy and “cut” the band, or you can perform an encircling buckle procedure to shorten the band.
RD Summary
Retinal detachments were once universally blinding, but with modern surgical techniques, sight can now be saved. If you suspect a retinal detachment in your patients, send them to an ophthalmologist right away as their prognosis depends upon the speed in seeking treatment.
ARMD
ARMD stands for Age Related Macular Degeneration and is a common retinal finding in older patients. ARMD is actually the leading cause of blindness in the elderly, at least in developed countries like the USA.
These patients develop extracellular breakdown deposits called “drusen” that form deep in Bruch’s membrane. Bruch’s membrane is the thin layer that separates the RPE/Retina from the underlying choroidal blood supply. This blockage keeps nutrition from percolating up from the choroid to the retina, and conversely, blocks photoreceptor waste products from draining down into the choroidal bed.
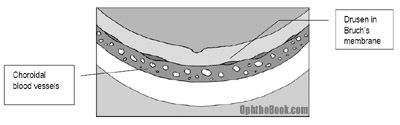
On exam you see localized retinal atrophy and pigmentary changes in the macula that correlate with poor central vision. The visual loss occurs slowly, however, and takes many years to progress.
Neovascular “wet” ARMD
If a break occurs in Bruchs membrane, vessels can grow up out of the deep choroidal circulation directly up into the retina! This is dangerous, as this neovascularization can bleed, create edema, and rapidly destroy vision.
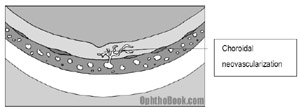
Treating this macular neovascularization is tricky – we would love to burn it away with a laser, but those bad blood vessels are often right at the fovea, and you don’t want to burn away central vision! Instead, we can use a few other techniques with variable success:
PDT (photodynamic therapy): With PDT, you inject a special chemical into the blood that reacts to specific wavelengths of light. Once the chemical floats within the retinal blood vessels, we then focus light of that desired wavelength directly at the fovea to coagulate the blood vessels without destroying the retina around it. Sounds good in theory, but it doesn’t work that well and is not as often done these days.
Lucentis: You can also inject anti-VEGF drugs like Avastin or Lucentis into the eye to stop angiogenesis. These anti-neovascular drugs also decrease vessel wall leakage and can help with other causes of macular edema.
Monitoring progression
Early, dry ARMD is very common and requires no treatment (other than possibly antioxidant vitamins), but we want to monitor these patients for progression to wet-ARMD. Patients can monitor themselves with an Amsler grid — a sheet of straight lines they can look at weekly for new metamorphopsia (distorted lines that might indicate macular edema).
Risk Factors?
So who gets ARMD? This disease occurs most often in elderly Caucasians with a positive family history for the condition. It’s almost always bilateral. Disease progression is also highly associated with smoking.
PIMP QUESTIONS
1. What is diabetic retinopathy, and by what mechanism does it occur?
This is retinal bleeding, edema, ischemia, and ultimately neovascularization caused by diabetic damage to the retinal blood vessels.
2. What are the retinal signs of diabetic retinopathy. How do they compare to, say, hypertensive retinopathy.
With diabetic retinopathy you typically see a lot of dot-blot hemorrhages, cotton-wool spots, and hard exudates. Hypertension usually has more flame hemorrhages and vascular changes such as arterial-venous nicking and copper/silver wiring.
3. How are angiogenic molecules involved with diabetic retinas?
VEGF production by areas of ischemic retina leads to neovascularization. These new vessels are bad as they can cause traction, bleeding, detachments, etc..
4. How do we categorize diabetic retinopathy?
As either NPDR (nonproliferative diabetic retinopathy) or PDR (proliferative diabetic retinopathy) depending upon the presence of neovascularization.
5. What are some mechanisms in diabetic retinopathy that might lead to decreased vision? What causes the majority of vision loss in diabetic patients?
There are several mechanisms for potential vision loss in these patients, including:
Macular edema (probably the leading cause of vision loss)
Vitreous hemorrhage
Retinal detachment
6. How do we treat advanced diabetic retinopathy?
Proliferative diabetic retinopathy is treated with PRP (pan retinal photocoagulation). By ablating the peripheral ischemic retina with a laser, we decrease VEGF production and thus decrease neovascularization.
7. A 35 year old man with bad type-1 diabetes presents with a pressure of 65. His anterior chamber is deep but you find neovascularization everywhere – in the retina and on the iris. What do you think is causing the pressure rise, and how do you treat it?
The pressure is up because of neovascularization of the iris angle with blood vessels clogging up the trabecular drain. You treat neovascularization by PRP lasering the peripheral retina to decrease VEGF production. NVA (neovascularization of the angle) is hard to manage and this patient will probably require a surgical drainage procedure in the near future.
8. Describe the three types of retinal detachment?
These include rhegmatogenous detachments, tractional detachments, and exudative detachments.
9. What are the symptoms of a retinal tear or detachment?
Flashes and floaters are the classic signs. With a large detachment your patient may also notice an area of “dark curtain” or “blurry spot” in their peripheral vision.
10. What is a PVD?
This is a posterior vitreous detachment – with aging the vitreous jelly liquefies and contracts. A sudden contraction can cause new floaters. This event is usually harmless, but you should search carefully for retinal tears.
11. An elderly patient presents with a brief episode of flashing and now has a single floater that moves with eye movement. A thorough retina exam reveals no detachment or tear, but you observe a small vitreous opacity floating over the optic disk. What has happened?
This again sounds like a PVD. The floater is a Weis ring, a piece of optic disk debris that has pulled off with the detachment. PVDs are common and usually harmless, though patients should have a thorough exam for retinal tears and be taught about the symptoms of retinal detachment to look out for.
12. A patient presents late at night with a large bullous retinal detachment. The central fovea is also detached. How soon do you need to go to surgery?
If the macula is off, then the macular photoreceptors are already damaged and it may be ok (this is the retina surgeon’s call) to schedule repair later when your surgeon is well-rested and you’ve got your best operating team. However, if the macula is still ON, you want to intervene sooner to make sure the macula STAYS on.
13. What kind of surgeries can we perform to relieve retinal detachments?
You can perform a vitrectomy to clean out the inside of the eye and relieve retinal traction. While in there you can also reappose the retina. You can also perform a scleral buckle or a pneumatic retinopexy.
14. What is Schafer’s Sign?
This is when you see retinal pigment particles floating in the anterior vitreous chamber behind the lens. This slit-lamp sign increases your suspicion for a tear or detachment.
15. What kind of travel restrictions would you tell a patient who has a pneumatic retinopexy?
Well, you don’t want these patients to fly. A decrease in ambient pressure causes gases to expand. If this happens in the eye it could explode! Your patients should also avoid SCUBA diving for similar reasons, as the change in gas volume over the changing atmospheric pressure will cause extreme pain and possible damage.
16. What’s the difference between dry and wet age-related macular degeneration?
Dry ARMD is when you have drusen and macular RPE atrophy. Wet ARMD implies choroidal neovascularization that has grown up through Bruch’s membrane.
Advise MEDICAL STUDENTS & ALL OPHTHA RESIDENTS TO READ IN EARLY PRACTICE.
Dr.M.FRGHLY,OPHTHALMOLOGIST.
you are doing a great swervice to the medical profession
wt is RPE stands for?
Editors Note: Retinal Pigment Epithelium. This is the pigmented layer immediately underneath the photoreceptors, that seperates the retina from the underlying choroidal blood supply.
Just awesome work doctor
…
it is usefull in wards rapid review and study as u know they give us just 2 weeks for eye !
can i ask if any body just know something cool just like this handsome work by doctor tim ..but for ENT as my ENT wards just started ..!!
Thank you so very much.
thank you
Thank you thank you thank you! EVERY subspecialty needs a site like this!
This book is special in that it tells me everything I wanted to know but embarrassed to ask as an eye SHO.
No other textbook does that! Thanks Dr Root!
PS: Can we ask for a chapter in oculoplastics?
Serena Park, SHO ophthalmology, Wellington Regional Hospital, Wellington, New Zealand
I am 2 days from medical finals – this has just saved my life!! Brilliant!
simply a great work doctor.thanks
Wow this is super cool. My medical school teaching is nothing compared to this! We get 5 days on opthalmology placement so this is a real help, and I shall be visiting this website alot in the run up to finals! Thank you very much! Sheffield, UK.
thnks alot , l like this site very much with its great animations,,really it helps me and i hope there will be rest of ophthalmogy lectures..thnks again dr 🙂
its really awesome! it made the subject so easy to understand. Thanks a ton to the doctors team for this great effort…….
u are the best…im so happy i found this site,mwah
all thngs r easy to understand in a simple way……really thankful…:-)
This is reallygood book .it’s helping me alot
can any one tel, y nasal arterioles r first involved in retinopathy of pregnancy induced hypertension?
thanks this is nice.
what is binocular vision.what tests can one perform in px with no binocular vision and how do one perform streak retinoscopy highlighting mixed astigmatism,high myopic astigmatism and aphakia.
PAUL NENANI BANDA(optometry student,zambia)
good
Very simple…. 2 days away from opth exam! Super helpful.. Thx u lots
Thanks for making it understandable for a patient.
Subject is mid 70’s and had been diagnosed with macular degeneration. New diagnosis of aneurysms behind both retinas with laser treatment plan. Should I ask for a second opinion?
thanks alot for making difficult parts easy to understand….
thaaaaaank you so so so so so much
Thank you Dr. Kindly help on retinal landmarks as its nowhere clear on the web…animations or diagrams will be an added help..thanks..
Thanks a lot! This was really amazing.. Easy to understand.
amazing donot deprive us from it
Thanks for giving such book and short but sweet ideas about retina.
Well written compilation of notes, makes eye disorders easier to understand.
cheers
can u explain squint ir strabismus …..not understandin fom any book
and ur book is tooo goood
at last i found a book so easy to understand thank you dr.root
book is really
THANKS ATON
Thanks a mill! you are a great man for making information freely available!!!
A year back we overcome the negative korioretiniti, avastin taken proper medical treatment, which was advised retina to improve vitamin?
Thanks a lot. Only God can repay your kindness. 🙂
Thanks so much for these I particularly like the video lectures they have helped me lots and have now made me very keen to do ophthalmology, (shame its so dam competitive!)
Really helpful, thanks.
hello doctor
My son 18 years now old met with an accident when he was 6 years old. he was ok after the accident no complain of eyes sight or any other complications. but at the age of 13 yeras he lost the hearing for both the ears and is supported by hearing aid .now he is reported as 30% loss damage to retina of his left eye and right eye is good. is there a treatment for this loss…is there a chance to retina damage of further loss,tell me doctor what treatment to be taken immmdiately
Awesome post.
Awesome. truly appreciated!
relle relle helpful videos…thnku Dr.Tim
Thank you Dr Root. Your explanation and animation are great. They have me understand very complex topics.
I love Root Eye Network. Fantastic !!!
Thanks Dr. Root…never realized there was so much
to learn regarding the eye!
I’m currently in PA school and this is GREAT information! I feel beyond prepared after reading this!
Great work. I am a budding diabetologist and planning to start retina.many thanks.
relly helpful n easy to understand… many thnkz
its a very good effort.easy and informative.having detail and basic knowledge.thanks a lot.
This is Awesome !! Appreciate your to-the-point, easy-to-understand explanation of concepts.
Thanks doc, this was really helpful.
What is the difference between a Lensectomy and a Pars Plana Lensectomy?
simple and easy to understand,
thank you.
i just joined eye unit and this is of great!
Thank you so much ,prof . it’s so helpful
Thanks Dr Tim. Your website has beem of great help to me